Home / Biotech
Discover our trajectory and what we do to guarantee access to highly complex medicines for all Brazilians. A pioneer company in the production and commercialization of innovative biological medicines and biosimilars.
We are a Brazilian company 100% dedicated to biotechnology. Founded in 2012, Bionovis is located in the city of Valinhos/SP and is a 100% national company that operates in the research, development, production, and commercialization of highly complex biopharmaceuticals. Always following the most rigorous scientific, technical, and ethical standards, we have the production capacity to serve not only the Brazilian market but also Latin America and other regulated countries.


Many milestones and significant partnerships have shaped us as a solid company, ensuring Bionovis’ mark in the history of biotechnology in Brazil. Explore all the important Productive Development Partnership (PDP) agreements signed on our journey.

Throughout our history, we have positively impacted around 1 million patients through the provision of more than 19 million vials and syringes of biopharmaceuticals.
We are committed to ensuring Brazil’s self-sufficiency in the development and manufacturing of high-quality, highly complex biological products, continuously expanding Brazilians’ access to these medicines.

Investments of more than 1,3 billion BRL in manufacturing infrastructure, highly qualified human resources, and technology acquisition.
Aligned with global standards and the requirements established by the Brazilian Health Regulatory Agency (Anvisa) and regulatory agencies in the United States (Food and Drug Administration – FDA) and Europe (European Medicines Agency – EMA).
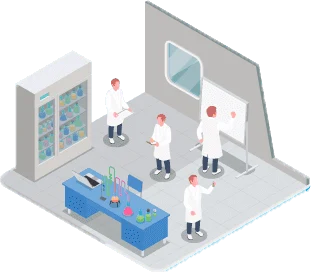
Fully equipped and operational, this is where the initial steps are taken to receive technology from partners, absorb and master cell cultivation processes, and develop the processing, formulation, and packaging of test batches for the production of biological medicines. The Bionovis pilot plant has the capacity to operate with two bioreactors of up to 200 liters
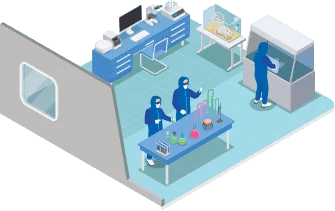
To guarantee quality and pharmacological properties within permitted parameters, Bionovis has a complex quality control laboratory. In this facility, the necessary tests are conducted to ensure that the manufactured products meet the technical and scientific criteria established by ANVISA and other important national and international regulatory agencies.
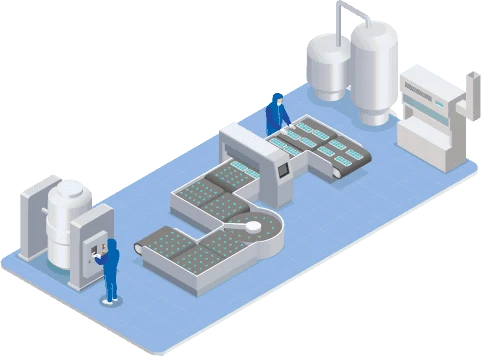
Our industrial plant has three lines of active ingredients for the industrial production of highly complex biopharmaceuticals: one with reactors operating in a continuous perfusion regime and two in a fed-batch regime, which use single-use reactors. The production lines are capable of operating with mammalian cells — a standard in the global biotechnology industry — using reactors with a total volume of more than 20,000 liters.
Another line is intended for final processing, for formulating, filling and packaging the product. The plant has the capacity to produce up to 250 kg of protein for biological medicines per year and allows the manufacture of ten highly complex biopharmaceuticals for the treatment of autoimmune and oncological diseases. This volume guarantees the supply of all domestic domestic demand, in addition to exports to other countries.
Bionovis is responsible for importing raw materials and biopharmaceuticals from international partners and ensuring proper storage and efficient logistics, so that the products reach patients with quality.


Ensuring high national and international quality standards is essential to obtain approvals for new products and maintain Bionovis‘ reliability with doctors, patients, partners, and regulatory agencies. Good medicine practices are related to manufacturing procedures that include fundamental requirements to ensure product efficacy and safety.
Bionovis has a series of certifications from ANVISA, such as medicine registration, operating authorization, inspection of pharmaceutical laboratories, and good Distribution and Storage practices. Furthermore, we are preparing to receive licenses from international bodies, which will further reinforce the good conduct adopted and the quality of our products.

Bionovis is a pioneer in the production and commercialization of biological and biosimilar medicines.
Our pursuit of scientific innovations and highly complex medicines aims to build a healthier Brazil — and a healthier world — for everyone.
© 2025 Bionovis. Companhia Brasileira de Biotecnologia Farmacêutica.